Understanding Medical Device Symbols: A Guide to ISO 15223-1 Standards & Beyond
Key Takeaways: Understanding Medical Device Symbols & ISO 15223-1
- What is ISO 15223-1? An international standard that establishes a universal language of symbols for medical device packaging and labels. It eliminates language barriers to ensure safe handling and regulatory compliance.
- Why Symbols Matter:
- Universal Safety: visual cues that are understood globally, regardless of language.
- Regulatory Compliance: ensures adherence to EU MDR, FDA, and international safety standards.
- Space Efficiency: allows manufacturers to convey critical data (like sterility or batch codes) on small labels.
- Common Manufacturing & ID Symbols:
- Manufacturer / Date: Identifies the maker (factory symbol), the date of manufacture, and the Use-by date.
- Traceability: Includes Batch Code (Lot) and Catalog Number (Ref) for precise identification.
- Regulatory: CE Marking (European conformity) and Authorized Representative (EC REP).
- Sterility & Hygiene Symbols:
- Sterile Indicators: Distinguishes between sterilization methods (Ethylene Oxide, Irradiation, Steam/Dry Heat, or Aseptic techniques).
- Single Use: "Do Not Re-use" and "Do Not Resterilise" symbols to prevent cross-contamination.
- Package Integrity: "Do Not Use if Package is Damaged" alerts users to potential contamination.
- Storage & Handling Symbols:
- Environmental Controls: Instructions to Keep Dry, Keep Away from Sunlight, and specific Temperature/Humidity Limits.
- Atmospheric Pressure: Defines safe pressure ranges for storage and transport.
- Special Category Symbols:
- Device Type: Identifies items as a Medical Device (MD), In Vitro Diagnostic (IVD), or Prescription Only (Rx Only).
- Protection: Icons indicating protection against viruses, bacteria, or dangerous chemicals.
Medical device labels are filled with symbols that convey critical information to healthcare providers, patients, and users. Symbols play a crucial role in medical device labelling, providing essential information quickly and universally. While many are familiar with symbols from ISO 15223-1, which are widely used for medical device labels and packaging, other important symbols also appear in healthcare products.
1. What is ISO 15223-1?
ISO 15223-1 is an international standard that defines symbols to be used with medical devices and associated information. These symbols are widely recognised and help reduce misunderstandings by providing clear, visual cues for device handling, safety, and performance.
2. Why are Symbols Important on Medical Devices?
Symbols on medical device labels allow for:
- Quick Understanding: Symbols communicate essential information without the need for language, making devices usable across global markets.
- Compliance and Safety: They ensure users follow regulatory requirements, protecting both users and patients from potential risks.
- Space Efficiency: Compact symbols save space on labels, allowing for more information to be conveyed in a concise manner.
3. Key Medical Device Symbols
Below are essential symbols commonly found on medical devices and packaging. These symbols are not limited to the ISO 15223-1 standard but also include other important regulatory and safety symbols.

Manufacturer
Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/ EEC and 98/79/EC.

Authorized Representative in the European Community
Indicates the Authorized representative in the European Community.

Date of Manufacture
Indicates the date when the medical device was manufactured.

Use-by Date
Indicates the date after which the medical device is not to be used.

Batch Code
Indicates the manufacturer’s batch code so that the batch or lot can be identified.

Catalog Number
Indicates the manufacturer’s catalog number so that the medical device can be identified.

Sterile
Indicates a medical device that has been subjected to a sterilisation process.

Sterilized Using Aseptic Processing Techniques
Indicates a medical device that has been manufactures using accepted aseptic techniques.

Sterilized Using Ethylene Oxide
Indicates a medical device that has been sterilized using ethylene oxide.

Sterilized Using Irradiation
Indicates a medical device that has been sterilized using irradiation.

Sterilized Using Steam or Dry Heat
Indicates a medical device that has been sterilized using dry heat.

Sterile Fluid Path
Indicates the presence of a sterile fluid path within the medical device in cases when other parts of the medical device, including the exterior, might not be supplied sterile.
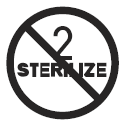
Do Not Resterilise
Indicates a medical device that is not to be resterilised.

Do Not Re-Use
Indicates a medical device that is intended for one single use only.

Non-sterile
Indicates a medical device that has not been sujected to a sterilization process.

Do Not Use if Package Is Damaged and Consult Instructions for Use
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information.
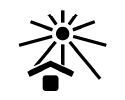
Keep Away from Sunlight
Indicates a medical device that needs protection from light sources.

Keep Dry
Indicates a medical device that needs to be protected from moisture.
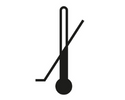
Lower Limit of Temperature
Indicates the lower limit of temperature to which the medical device can be safely exposed.
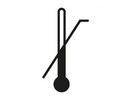
Upper Limit of Temperature
Indicates the upper limit of temperature to which the medical device can be safely exposed.
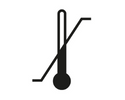
Temperature Limit
Indicates the temperature limits to which the medical device can be safely exposed.
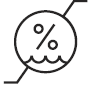
Humidity Limitation
Indicates the range of humidity to which the medical device can be safely exposed.
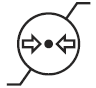
Atmospheric Pressure Limitation
Indicates the range of atmospheric pressure to which the medical device can be safely exposed.

Consult Instructions for Use or Consult Electronic Instructions for Use
Indicates the need for the user to consult the instructions for use.

CE Marking
Signifies European technical conformity.

Medical Device
Indicates the item is a medical device.

In Vitro Diagnostic Medical Device
Indicates a medical device that is intended to be used as an in vitro diagnostic medical device.

Prescription Device or Product
Federal law restricts this device to sale by or on the order of a licensed practitioner.
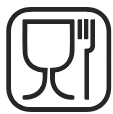
Food Safe
Indicates that the material used in the product is considered safe for food contact.

Virus Protection
Indicates that the medical device has protection against bacteria, fungi and viruses.
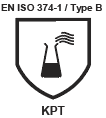
Chemical Protection
Indicates that the medical device has protection against certain dangerous chemicals.
4. How These Symbols Enhance Global Compliance
The use of standardized symbols, such as those from ISO 15223-1, the CE mark, and other recognized standards, ensures that medical devices meet international regulatory requirements, including those set by the EU Medical Device Regulation (MDR) and the U.S. FDA. This harmonization of symbols simplifies compliance across borders, reduces labelling complexities, and enhances user safety by offering clear, universal communication.
5. Conclusion
Medical device symbols, whether they originate from ISO 15223-1, the CE mark, or other global standards, play a crucial role in the healthcare industry by providing clear, standardized communication. Understanding these symbols is essential for anyone involved in the manufacturing, distribution, or use of medical devices to ensure both safety and regulatory compliance. Keep this guide as a handy reference for interpreting important symbols across various medical devices.
Looking for reliable medical products that meet international standards?
At Clearview Medical, we offer high-quality medical devices and supplies to support your business needs. Explore our range of products today and ensure your medical devices are fully compliant.
Browse our products or contact us for more information!


Understanding Medical Device Symbols